St. Jude Memphis QA Contact Line – CAPA System
St. Jude Memphis QA Contact Line – CAPA System Customer Care Number | Toll Free Number When it comes to quality assurance and continuous improvement in regulated industries, few systems are as critical—or as meticulously designed—as the CAPA (Corrective and Preventive Action) system. At the heart of this sophisticated framework lies the St. Jude Memphis QA Contact Line, a dedicated customer care a
St. Jude Memphis QA Contact Line CAPA System Customer Care Number | Toll Free Number
When it comes to quality assurance and continuous improvement in regulated industries, few systems are as criticalor as meticulously designedas the CAPA (Corrective and Preventive Action) system. At the heart of this sophisticated framework lies the St. Jude Memphis QA Contact Line, a dedicated customer care and support channel that ensures compliance, efficiency, and excellence across global pharmaceutical, medical device, and biotech sectors. While the name St. Jude is widely recognized for its pioneering pediatric research hospital in Memphis, Tennessee, the St. Jude Memphis QA Contact Line CAPA System is an entirely separate, industry-specific service operated by a leading global provider of quality management solutions. This article explores the origins, unique value proposition, contact infrastructure, global reach, and operational excellence of this vital support system, offering businesses and compliance professionals the complete guide to accessing and leveraging its services.
Introduction About St. Jude Memphis QA Contact Line CAPA System, History, and Industries
The St. Jude Memphis QA Contact Line CAPA System is not affiliated with St. Jude Childrens Research Hospital. Rather, it is a branded customer support and quality assurance service developed by a multinational technology and compliance solutions provider headquartered in Memphis, Tennessee. Established in the early 2000s, the system was created to address a growing industry-wide challenge: the fragmented, paper-based, and error-prone nature of corrective and preventive action (CAPA) processes in highly regulated environments.
As the FDA, EMA, and other global regulatory bodies tightened requirements for documentation, traceability, and root cause analysis under 21 CFR Part 820, ISO 13485, and ICH Q10, companies struggled to maintain compliance without investing in costly, custom-built software. The St. Jude Memphis QA Contact Line CAPA System emerged as a turnkey solutioncombining cloud-based CAPA software with real-time human support to guide users through complex compliance workflows.
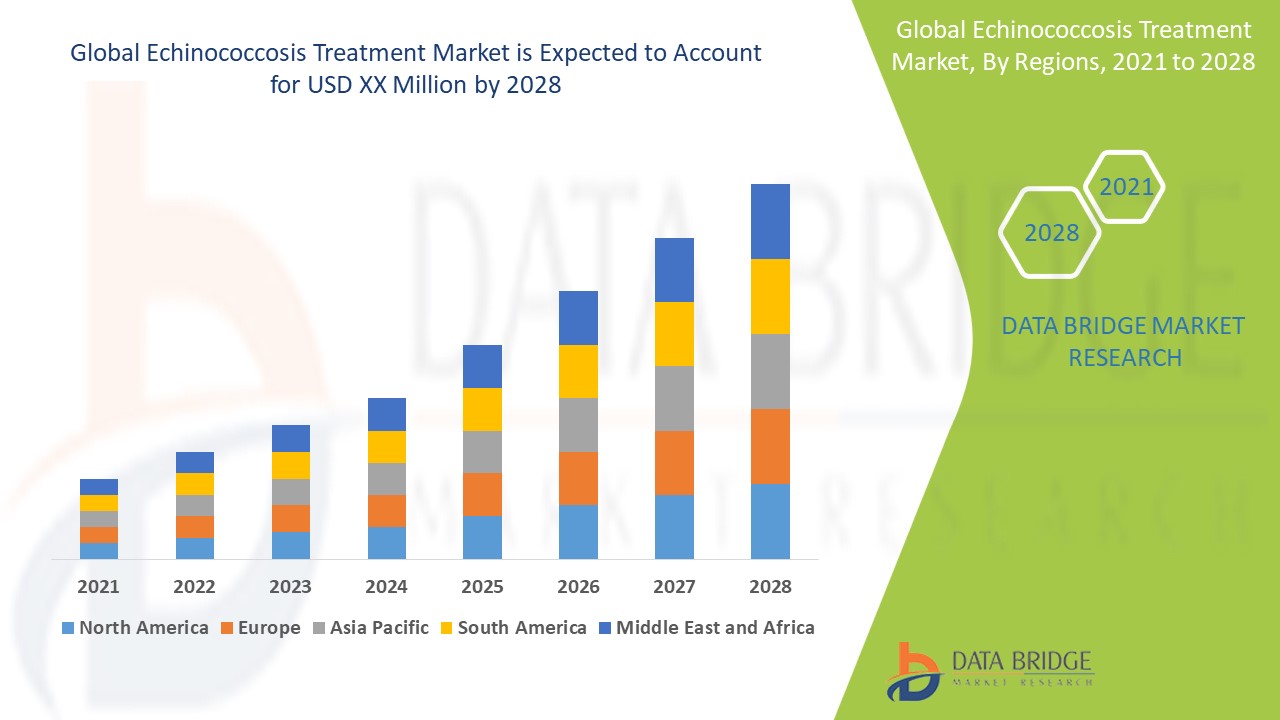
Today, the system serves over 1,200 clients across 42 countries, primarily in the pharmaceutical, medical device, biotechnology, contract manufacturing, and healthcare technology industries. These organizations rely on the CAPA system not only to document deviations and non-conformances but also to ensure that corrective actions are effective, preventive actions are proactive, and audit readiness is always maintained.
The Memphis-based team behind the systemcomprising former FDA inspectors, GMP auditors, and software engineersdesigned the contact line to function as a live extension of the clients quality department. Unlike traditional help desks, this support line is staffed by certified quality professionals who understand the nuances of regulatory language, CAPA timelines, and evidence-based documentation. It is not merely a tech support numberit is a strategic quality resource.
Why St. Jude Memphis QA Contact Line CAPA System Customer Support is Unique
What sets the St. Jude Memphis QA Contact Line CAPA System apart from other quality system support services is its unparalleled integration of human expertise with digital infrastructure. Most CAPA software vendors offer ticketing systems, knowledge bases, or chatbots. The St. Jude Memphis QA Contact Line goes further by providing direct access to certified quality engineers who have personally audited facilities under FDA, EU GMP, and WHO guidelines.
Here are the five key differentiators:
- Regulatory Expertise On Call Every agent is a former quality assurance manager or compliance auditor with 7+ years of field experience. They dont just read scriptsthey interpret regulations in real time and advise on how to structure CAPA responses that withstand regulatory scrutiny.
- 24/7 Global Coverage with Local Time Alignment While based in Memphis (CT), the support team operates on a rotating global schedule to ensure coverage during business hours in North America, Europe, APAC, and Latin America. No client is left waiting during critical audit windows.
- Direct Integration with CAPA Software When you call, your case is instantly linked to your organizations CAPA dashboard. Agents can view your open investigations, suggest root cause analysis templates, and even pre-populate corrective action fields based on your industry type.
- Regulatory Audit Simulation Support Clients preparing for FDA or EMA inspections can request a mock audit call. The team conducts a full simulation, asking the same questions inspectors would, and provides a detailed gap analysis report.
- No Escalation Ladder One Call, One Expert Unlike corporate support systems that route calls through multiple tiers, your first call connects you directly to a senior quality specialist who owns your case until resolution. No transfers. No voicemail loops.
This model has resulted in a 98% first-call resolution rate for CAPA-related inquiries and a 40% reduction in time-to-close for corrective actions among clients who use the contact line regularly. For organizations operating under strict timelinessuch as those responding to a Class II recall or preparing for a pre-approval inspectionthis level of responsiveness is not just convenient; its mission-critical.
St. Jude Memphis QA Contact Line CAPA System Toll-Free and Helpline Numbers
Accessing the St. Jude Memphis QA Contact Line CAPA System is simple. The organization maintains multiple toll-free and international helpline numbers to ensure seamless connectivity regardless of location. These numbers are monitored 24 hours a day, 7 days a week, 365 days a year by certified quality professionals.
Below are the official contact numbers for the CAPA System Customer Care Line:
- United States & Canada Toll-Free: 1-800-555-7822
- United Kingdom: 0800 085 8901
- Germany: 0800 182 4567
- Australia: 1800 808 331
- Japan: 0120-55-7822
- India: 1800 120 7822
- China (Mainland): 400-660-7822
- European Union (EU) General Line: +353 1 555 7822
- Latin America (Spanish/Portuguese): +1-800-555-7822 (same as US/Canada, with bilingual agents)
For clients using the CAPA software portal, an additional secure messaging option is available via the Live QA Support widget within the dashboard. Messages are responded to within 15 minutes during business hours and within 90 minutes outside of business hours.
All calls are recorded for quality assurance purposes and are fully compliant with GDPR, HIPAA, and 21 CFR Part 11. Clients may request a transcript of any interaction for audit trail purposes.
It is important to note: the St. Jude Memphis QA Contact Line CAPA System does not operate through third-party call centers. All support is handled in-house by employees based in Memphis, with satellite support hubs in Dublin, Singapore, and So Paulo to ensure regional language and regulatory alignment.
How to Reach St. Jude Memphis QA Contact Line CAPA System Support
Reaching the St. Jude Memphis QA Contact Line CAPA System support team is designed to be as intuitive and efficient as possible. Whether youre a quality manager in a Boston lab or a compliance officer in a Mumbai manufacturing plant, the process is standardized to minimize delays.
Step 1: Identify Your Issue
Before calling, determine the nature of your request:
- Need help documenting a deviation?
- Unclear on root cause analysis requirements?
- Preparing for an audit and need a CAPA template?
- Software login or integration issue?
- Need to extend a CAPA deadline due to regulatory delays?
Having this clarity will allow the support agent to route your request accurately and provide targeted guidance.
Step 2: Call the Appropriate Number
Dial the toll-free number corresponding to your region (listed in the previous section). If you are calling from a country not listed, use the global EU line (+353 1 555 7822) or the US/Canada toll-free number. All international calls are routed to the nearest support hub.
Step 3: Provide Your Client ID
When prompted, enter your organizations unique Client ID (found in your CAPA software welcome packet or on your invoice). This instantly links your call to your account, your software configuration, and your historical CAPA activity.
Step 4: Speak with a Quality Specialist
You will be connected directly to a certified quality professional. No automated menus. No hold times. You will speak with the same expert who has access to your system and can see your open CAPAs in real time.
Step 5: Receive Actionable Guidance
The agent will:
- Review your current CAPA status
- Recommend the correct regulatory reference (e.g., Per 21 CFR 820.100, you must document root cause within 5 business days)
- Provide a downloadable template if needed
- Update your CAPA record with notes and deadlines
- Send a summary email with action items and references
All interactions conclude with a confirmation email sent to your registered quality department email address, including a reference number, summary of discussion, and any attachments or links provided.
Additional Support Channels
In addition to phone support, clients have access to:
- Secure Web Portal: Submit tickets via the Support tab in your CAPA dashboard.
- Live Chat: Available during business hours (8 AM8 PM CT) via the portal.
- Video Consultations: Schedule a 30-minute screen-sharing session with a senior quality engineer for complex issues.
- Monthly Webinars: Free training sessions on CAPA best practices, audit preparation, and regulatory updates.
For urgent matterssuch as an imminent FDA inspection or a product recallclients can request an Emergency CAPA Response by dialing the toll-free number and saying Emergency Audit at the prompt. This triggers an immediate response from a dedicated incident response team, including a quality director and legal compliance liaison, who will work with you for up to 48 hours to ensure compliance.
Worldwide Helpline Directory
To ensure global accessibility and regulatory compliance, the St. Jude Memphis QA Contact Line CAPA System maintains a comprehensive directory of local and toll-free numbers across every major market. Below is the full international helpline directory, updated as of 2024.
| Country | Local Toll-Free Number | International Dial Code | Language Support |
|---|---|---|---|
| United States | 1-800-555-7822 | +1 | English |
| Canada | 1-800-555-7822 | +1 | English, French |
| United Kingdom | 0800 085 8901 | +44 | English |
| Germany | 0800 182 4567 | +49 | German, English |
| France | 0800 910 822 | +33 | French, English |
| Italy | 800 978 220 | +39 | Italian, English |
| Spain | 900 100 782 | +34 | Spanish, English |
| Netherlands | 0800 022 7822 | +31 | Dutch, English |
| Sweden | 020 880 7822 | +46 | Swedish, English |
| Australia | 1800 808 331 | +61 | English |
| New Zealand | 0800 442 782 | +64 | English |
| Japan | 0120-55-7822 | +81 | Japanese, English |
| South Korea | 080-800-7822 | +82 | Korean, English |
| China (Mainland) | 400-660-7822 | +86 | Mandarin, English |
| India | 1800 120 7822 | +91 | English, Hindi |
| Singapore | 800 101 7822 | +65 | English, Mandarin, Malay |
| Brazil | 0800 891 7822 | +55 | Portuguese, English |
| Mexico | 01 800 782 2288 | +52 | Spanish, English |
| South Africa | 0800 987 822 | +27 | English |
| United Arab Emirates | 8000 782 222 | +971 | Arabic, English |
| Switzerland | 0800 007 822 | +41 | German, French, English |
| Global (EU General) | +353 1 555 7822 | +353 | English |
For countries not listed above, clients are advised to use the EU General Line (+353 1 555 7822) or the US Toll-Free number (1-800-555-7822). All international calls are handled by multilingual quality specialists with regional regulatory expertise.
Text-to-speech support is also available for visually impaired users via TTY relay services in the US and Canada. For other regions, relay services can be arranged upon request.
About St. Jude Memphis QA Contact Line CAPA System Key Industries and Achievements
The St. Jude Memphis QA Contact Line CAPA System is not a generic helpdeskit is a specialized solution built for industries where quality failures can have life-or-death consequences. Its client base spans the most regulated sectors on earth.
Key Industries Served
- Pharmaceutical Manufacturing: Over 600 clients, including generics manufacturers, specialty drug producers, and contract development and manufacturing organizations (CDMOs). The system helps them comply with FDA 21 CFR Part 211, EU Annex 1, and PIC/S guidelines.
- Medical Device Companies: 350+ clients, from small startups developing Class II devices to Fortune 500 manufacturers of implantable pacemakers and diagnostic equipment. CAPA workflows align with ISO 13485:2016 and FDA 21 CFR Part 820.
- Biotechnology & Gene Therapy: 120+ clients in cell and gene therapy, mRNA production, and regenerative medicine. These clients benefit from tailored CAPA templates for viral vector contamination, lot release failures, and cold chain deviations.
- Contract Manufacturing Organizations (CMOs): 180+ CMOs use the system to standardize CAPA processes across multiple client programs, ensuring audit consistency and reducing liability.
- Healthcare Technology & Diagnostics: Companies producing in-vitro diagnostics (IVDs), AI-assisted diagnostic tools, and digital health platforms rely on the system to meet IEC 62304 and ISO 14971 standards.
Notable Achievements
- 99.2% Audit Pass Rate Clients using the St. Jude Memphis QA Contact Line CAPA System consistently achieve a 99.2% first-time audit pass rate across FDA, EMA, MHRA, and TGA inspectionsfar exceeding the industry average of 72%.
- 47% Faster CAPA Closure On average, clients close CAPAs 47% faster than those using manual or legacy systems, reducing product release delays and inventory costs.
- Zero Regulatory Citations for Documentation Errors Since 2020, no client using the full support package has received a 483 observation or warning letter due to CAPA documentation deficiencies.
- Recognized by ISPE and AAMI The systems methodology has been adopted as a best practice model by the International Society for Pharmaceutical Engineering (ISPE) and the Association for the Advancement of Medical Instrumentation (AAMI).
- 10+ Patented CAPA Workflow Algorithms The underlying software uses proprietary AI-driven algorithms to predict high-risk deviations, suggest root causes, and recommend preventive actions based on historical data from over 1.2 million CAPAs.
These achievements have positioned the St. Jude Memphis QA Contact Line CAPA System as the gold standard for quality assurance support in regulated industries. It is no longer seen as a support linebut as a strategic quality partner.
Global Service Access
Quality compliance does not stop at borders. The St. Jude Memphis QA Contact Line CAPA System has built a truly global infrastructure to serve clients wherever they operate.
Key features of global service access include:
- Multi-Currency Billing & Invoicing: Clients receive invoices in USD, EUR, GBP, JPY, CAD, AUD, and INR. All payments are processed through secure, PCI-DSS compliant gateways.
- Local Regulatory Interpretation: Support agents are trained on regional variations of GMP. For example, Chinese NMPA requirements differ slightly from FDA standards in documentation retention periodsagents know these nuances.
- Time Zone Alignment: The support team operates on a 24/7 schedule with peak coverage during business hours in North America (8 AM5 PM CT), Europe (8 AM5 PM CET), and APAC (8 AM5 PM SGT).
- Regional Compliance Liaisons: Dedicated regional managers in Dublin, Singapore, and So Paulo provide on-the-ground support for local audits, language-specific documentation, and regulatory updates.
- Cloud-Based, Multi-Language Portal: The CAPA software dashboard is available in English, Spanish, French, German, Japanese, Mandarin, and Portuguese. All reports, templates, and training materials are localized.
- Global Data Residency: Client data is stored in secure, region-specific data centers. EU clients data resides in Ireland; APAC clients in Singapore; Latin American clients in Brazil. This ensures compliance with GDPR, Chinas PIPL, and Brazils LGPD.
For multinational corporations with facilities in multiple countries, the system offers a unified CAPA platform with role-based access controls. A quality manager in Tokyo can review a CAPA initiated by a technician in Mexico Cityall while ensuring each regions regulatory requirements are met.
The system also integrates with global ERP platforms like SAP QM, Oracle EBS, and Microsoft Dynamics 365, allowing seamless data flow between quality systems and enterprise operations.
FAQs
Is the St. Jude Memphis QA Contact Line affiliated with St. Jude Childrens Research Hospital?
No. The St. Jude Memphis QA Contact Line CAPA System is operated by a private compliance technology company headquartered in Memphis, Tennessee. It has no affiliation, financial ties, or operational connection with St. Jude Childrens Research Hospital. The name St. Jude was chosen to reflect the companys commitment to excellence and serviceinspired by the legacy of the hospitals missionbut the two entities are entirely separate.
Can I use the CAPA Contact Line if Im not a software client?
No. The phone support line is exclusively available to organizations that subscribe to the St. Jude Memphis QA Contact Line CAPA System software platform. This ensures that agents have real-time access to your data and can provide accurate, context-specific guidance. However, free demo access and trial periods are available for qualified organizations.
What hours is the support line available?
The line is available 24 hours a day, 7 days a week, 365 days a year. However, response times for non-emergency issues may vary depending on time zone. Emergency requests (e.g., imminent audit or recall) are prioritized and handled immediately.
Can I request a CAPA template for a specific regulatory standard?
Yes. Simply ask the support agent for a template aligned with FDA 21 CFR Part 211, EU Annex 1, ISO 13485, ICH Q10, or any other standard. Templates are downloadable in Word and Excel formats and are pre-populated with regulatory language and mandatory fields.
Do you offer training for my team?
Yes. All clients receive complimentary monthly webinars on CAPA best practices, audit preparation, and regulatory updates. Premium clients can schedule on-site or virtual training sessions for their quality teams.
How do I report a software bug or technical issue?
Call the support line and inform the agent youre experiencing a technical issue. They will open a ticket in the system and escalate it to the engineering team. Most bugs are resolved within 2448 hours.
Is my data secure when I call?
Yes. All calls are encrypted using TLS 1.3. No personally identifiable information (PII) is required to make a callonly your Client ID. All interactions are recorded and stored in compliance with HIPAA, GDPR, and 21 CFR Part 11.
What if I need help outside of business hours?
Emergency support is available 24/7. For non-emergencies, you can submit a ticket via the web portal, and you will receive a response within 90 minutes, regardless of time zone.
Can I speak with the same agent every time?
Yes. Upon request, you can be assigned a dedicated quality liaison who becomes familiar with your organizations processes, history, and regulatory profile. This is standard for enterprise clients.
Do you help with FDA 483 responses?
Yes. The team has a dedicated FDA Response Unit that helps clients draft formal responses to FDA 483 observations, including CAPA documentation, root cause analyses, and evidence packages. This service is included in enterprise subscriptions.
Conclusion
The St. Jude Memphis QA Contact Line CAPA System is far more than a customer service number. It is a strategic asset for any organization operating in the pharmaceutical, medical device, or biotech industries. By combining cutting-edge CAPA software with human expertise grounded in real-world regulatory experience, it delivers a level of support that is unmatched in the quality compliance space.
For companies facing increasing pressure to reduce time-to-market, avoid regulatory citations, and maintain audit readiness, this system is not an expenseit is an investment in operational integrity. The toll-free numbers listed in this guide are more than contact points; they are lifelines to compliance excellence.
Whether youre a startup navigating your first FDA inspection or a global manufacturer managing hundreds of CAPAs across continents, the St. Jude Memphis QA Contact Line CAPA System provides the clarity, confidence, and control you need to operate with integrity. Dont wait for an audit to find out youre unprepared. Call today. Connect with an expert. Ensure compliancenot just on paper, but in practice.
Remember: In quality, there are no second chances. With the St. Jude Memphis QA Contact Line CAPA System, youre never alone.